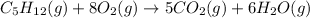Assuming the reactions are for complete combustion, the products are always carbon dioxide and water, so the unbalanced equation is:
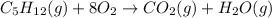
Now, we need to balance it.
As we can see, the carbon atom, C, only appears in one place on both sides, so let's start by balancing it.
We have 5 carbon on the left side, and one carbon of the right side, so we can balance the carbon atom by adding a coefficient of 5 on CO₂:
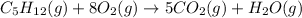
Now, we can balance the hydrogen atom, H. We have 12 hydrogen on the left side and 2 per water molecule on the right side. So, we can put the coefficient 6 on the water, so we balance the H atom:
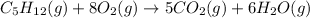
Now, we need to check wheter the oxygen atom, O, is balanced.
On the left side, we have 8 times 2, so 16 oxygen.
On the right side we have 5 times 2 on CO₂, so 10, plus 6 on the water, so a total of 16 oxygen.
Since both side are equal, the equation is balanced.
So, the final equation is: