The first step is to use the molecular weight of HCl to convert 145g to moles of HCl (mw=36.458g/mol):
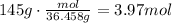
Now, use the ratio of the coefficients of MnCl2 to HCl, to find how many moles of MnCl2 are produced with this amount of HCl:
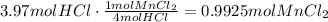
Use the molecular weight of MnCl2 to convert the amount of moles produced to grams:
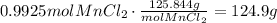
124.9g of MnCl2 are produced.