Answer:
Group 4A (Group IVA)
The answer is B
Step-by-step explanation:
An element with valence electron configuration:
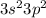
has 4 valence electrons.
Since the valence shell is n = 3 it is in Period 3. Since there are 4 valence electrons (2 in 3s and 2 in 3p) it is in Group 4A