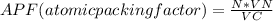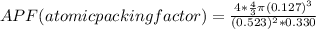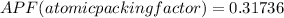Answer:
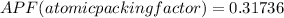
Step-by-step explanation:
From the question we are told that
Density of metal
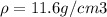
Atomic weight of
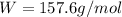
Atomic radius of
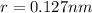
Lattice parameters=>
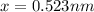 and
and
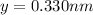
Generally the equation for atomic packing factor is mathematically given as
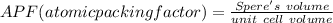
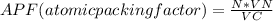
Generally the equation for number of atoms N is mathematically given as
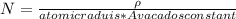
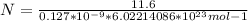
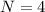
Therefore APF(atomic packing factor)