Answer:
518 grams
Explanations:
The reaction between magnesium and lead(IV)nitrate is given as:
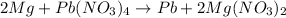
Given the following parameters
moles of aqueous lead (IV) nitrate = 2.50moles
According to stoichiometry, 1 mole of lead(IV)nitrate produce 1 mole of solid lead, the moles of lead required will be 2.50moles
Determine the mass of solid lead product
Mass of Pb = moles * molar mass
mass of Pb = 2.5 * 207.2
Mass of Pb = 518grams
Hence the mass of dried solid product that should be formed is 518 grams