Considering the Avogadro number, we know that there is 6,022 x 10^23 molecules of NaOH in 1 mol of NaOH, and we need to calculate how much in mols we would have for 2.90 ×10^22 molecules:
6,022 x 10^23 molecules ------- 1 mol
2.90 ×10^22 molecules ---------- x
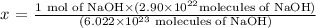
Solving for x, we have 0.048 mol of NaOH.
Now we calculate how much, in mass, 0.048 mol of NaOH is:
1 mol ---------- 40.0 g
0.048 mol ---------- y
Solving for y, we have 1.92 g of NaOH