Answer:
pH= 14.17
pOH= -0.17
[H3O+]= 6.76x10^-15M
[OH-]= 1.5M
Step-by-step explanation:
1st) It is necessary to write the dissociation equation:
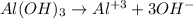
From the equation we can see that from 1 mole of Al(OH)3, 3 moles of OH- are dissociated. So, in this case, from the 0.5M solution, 1.5M of OH- will be produced (3x0.5M=1.5M).
We know that the concentration of OH- is [OH-]= 1.5M.
2nd) With the [OH-] we can calculate the pOH of the solution:
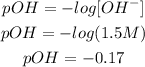
The pOH is -0.17.
3rd) Now we can calculate the pH of the solution:
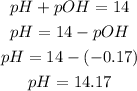
The pH is 14.17.
4th) Finally, we can calculate the concentration of H+:
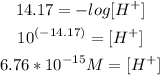
So, the concentration of H+ is 6.76x10^-15M.