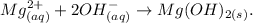Answer:
![Mg_((aq))^(+2)+2OH_((aq))^-\operatorname{\rightarrow}Mg(OH)_(2(s)).]()
Step-by-step explanation:
Remember that a net ionic equation is the chemical equation that shows only those elements, compounds, and ions that are directly involved in the chemical reaction.
First, let's state the balanced chemical equation:
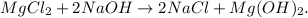
To do the net ionic equation, we just have to 'separate' each ion of each reactant and those ones in the product that don't bond to the OH, like this:
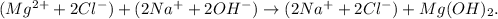
The final step is to 'cancel' the ions that are repeated such as Cl (-) and Na (+), the net ionic equation would be with their respective phases: