Answer:
1462.5Joules
Explanations:
The formula for calculating the amount of heat gained is expressed as:
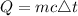
where:
• m is the mass of the ball of iron = 250.0g
,
• c is the specific heat capacity = 0.450J/gC
,
• △t is the change in temperature = 32 - 19 = 13oC
Substitute the given parameters
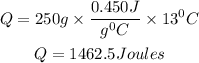
Therefore the amount of heat the ball gained is 1462.5Joules