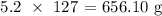Answer:
Step-by-step explanation:
Here, we want to convert the given number of atoms to grams
We start by getting the number of moles
Mathematically:
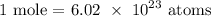
Now, let us get the number of moles in the given number of atoms
We have that as:

Now, to get the mass, we have to multiply the above number of moles by the atomic mass of iodine
The atomic mass of iodine is 127 atomic mass units
Thus, we have the mass as: