The Ideal Gas Law
There are some important equations that we must know:

- P = pressure
- V = volume
- n = moles
- R = Universal Gas Constant (8.31)
- T = temperature

Solving the Question
We're given:
- molar mass of O2 = 32.00 g/mol
- mass of O2 = 128 g
- T = 273 K (STP)
- P = 101.3 kPa (STP)
First, solve for n:


Now, solve using the Ideal Gas Law:

⇒ Isolate V:
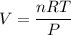
⇒ Plug in given values:

Answer
a. 89.6 L