Final answer:
Adding solid zinc to a copper(II) sulfate solution results in a redox reaction, forming metallic copper and zinc sulfate, and presenting as very fine, black copper particles. Alternatively, zinc in hydrochloric acid forms zinc chloride and hydrogen gas.
Step-by-step explanation:
When solid zinc metal is added to a dissolved penny solution, which typically contains copper(II) sulfate, a redox reaction occurs. This reaction involves the zinc metal dissolving to form Zn2+ ions while simultaneously reducing the Cu2+ ions to metallic copper. Observations during this process include the appearance of very fine particles of copper that may look black due to their fine size rather than the usual reddish color.
The relevant balanced chemical equation for this redox reaction is: Zn(s) +
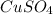 (aq) → Cu(s) +
(aq) → Cu(s) +
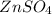 (aq). If the penny solution was a hydrochloric acid solution, the zinc would react to form zinc chloride and hydrogen gas as depicted in the reaction: Zn(s) + 2 HCl(aq) →
(aq). If the penny solution was a hydrochloric acid solution, the zinc would react to form zinc chloride and hydrogen gas as depicted in the reaction: Zn(s) + 2 HCl(aq) →
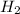 (g) +
(g) +
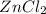 (aq).
(aq).