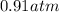Answer:
The pressure of the gas will be
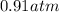
Step-by-step explanation:
At STP, the temperature of the system is
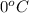 while the pressure is
while the pressure is
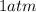
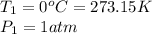
The temperature is changed to
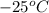 , thus
, thus
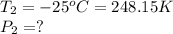
As volume is constant i.e.,
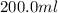 , by applying Gay-Lusaac law,
, by applying Gay-Lusaac law,
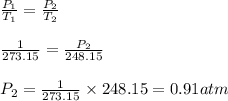
Thus, the pressure of
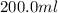 gas at
gas at
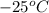 is
is