Answer
5.48 grams of C
Step-by-step explanation
Given:
Mass of Fe₂O₃ that reacted = 24.3 g
Equation: Fe₂O₃(s) + 3C(s) → 2Fe(s) + 3CO(g)
What to find:
The grams of C that are required to react with 24.3 g of Fe₂O₃(s).
Step-by-step solution:
According to the equation; 1 mole of Fe₂O₃(s) reacts with 3 moles of C
1 mole of Fe₂O₃ = 159.69 g
1 mole of C = 12.011 g, so 3 moles of C = 3 x 12.011 g = 36.033 g
Therefore, the mass of C required to react with 24.3 g of Fe₂O₃(s) is calculated as follows:
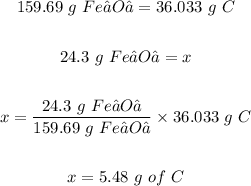
Thus, 5.48 grams of C are required to react with 24.3 g of Fe₂O₃(s).