We have the following balanced equation:
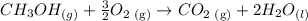
They also give us the heat of reaction equal to -764 kJ, i.e. it is an exothermic reaction.
By observing the reaction, we can deduce that for this heat to be generated, one mole of methanol is needed. Now let's see how many grams that mole of methanol equals. We will use the molecular weight equal to 32.04 g/mol
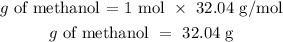
Now we know the grams of methanol that generate 764 kJ, because the heat of reaction is directly proportional to the mass of the reactants, we can apply a rule of three to know the grams needed to produce a heat of reaction equal to 701 kJ:
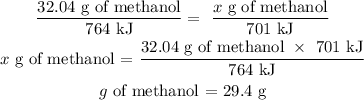
So, 29.4 g of methanol must be found to produce 701 kJ of heat