Chemistry => The Mole => Percent of water in a Hydrate
We have a compound that is hydrated with "n" moles of water. To determine the moles of water, what we will do is take as a base a mole of the dry compound, that is, 1 mol of Na2SO4.
We will determine the mass of one mol of the dry compound and the difference between the hydrated compound and the dry compound will be the water content.
Let's see what is the molar mass of the dry compound: Na2SO4
Element Mass
Na 2 x 22.99 =45.98 g/mol
S 1 x 32.065 = 32.065 g/mol
O 4 x 15.999 = 63.996 g/mol
Sum 142.04 g/mol
So, we have that one mol of Na2SO4 has a mass of 142.04.
Now, the water in 1 mol of the hydrated compound will be:
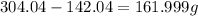
Now, we divide the mass obtained by the molar mass of water, 18.01 g/mol:
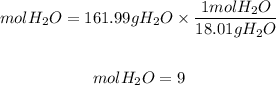
In the hydrated compound, we have 9 moles of H2O for each mol of Na2SO4.
Therefore the ANSWER will be B. 9