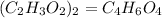Answer
C₄H₆O₄
Step-by-step explanation
Given:
The empirical formula of the compound is C₂H₃O₂
The molecular mass of the compound = 118.054 g/mol
To get the molecular formula of the compound, you need to know the actual number of atoms of each element by equating the empirical formula to the molecular mass as follows:
From the Periodic Table:
Molar mass of H = 1.00784 g/mol
Molar mass of O = 15.999 g/mol
Molar mass of C = 12.0107 g/mol

Hence, the molecular formula of the compound is: