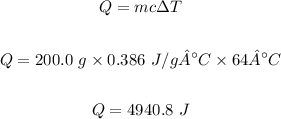Answer
Q = 4940.8 J
Step-by-step explanation
Given:
Mass of the copper sample, m = 200.0 grams
Change in temperature, ΔT = 64 °C
Specific heat of Cu, c = 0.386 J/g°C
What to find:
The energy needed, Q
Step-by-step solution:
Using the given equation, the energy needed can be calculated.