First, we will write the formula we will be using: the Ideal gas law

Now, let's see what is given to us
Volume: 28 Cubic Inches
Pressure: 35 PSI
New Volume: 5 cubic inches
New Pressure?
No change in temperature
Since we know that only the volume is changing, we can simplify the ideal gas law into Boyle's law
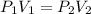
So now, we can subsititute in the values we got.
(28)(35) = 5(P2)
P2 = 196 PSI