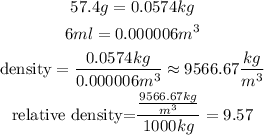Take into account that density and relative density are given by:
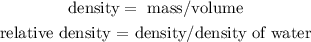
Take into account that the volume associated to each of the given sustances in the table is determined by the Level Difference (because it is the change in the volume of the water of the recipient in which the substance is immersed).
The density of water in kg/m^3 is 1000 kg/m^3.
Due to the density must be given in kg/m^3, it is necessary to express the volumes of the table in m^3 and mass in kg, then, consider the following conversion factor:
1 m^3 = 1000000 ml
1 kg = 1000 g
Then, you obtain the following results:
Brass:
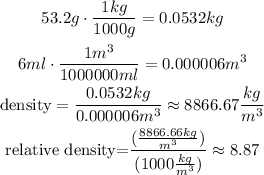
Cooper: