The amount of a radioactive element over time can be written as:
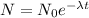
So, considering that we know the time of its half life, we know how long it takes to get to half the original amount. This is (usint the amount of days in microseconds):
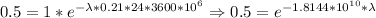
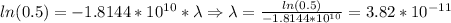
Thus, lambda=3.82*10^(-11)/microseconds