Given,
The gas sample is at STP.
Thus, the temperature of the gas, T=273.15 K
The pressure of the gas, P=10⁵ Pa
The quantity of the gas, n=2.3 mol
The value of the gas constant is R=8.314 m³·Pa·K⁻¹·mol⁻¹
From the ideal gas equation,
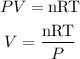
Where V is the volume of the gas.
On substituting the known values,
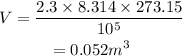
Thus the volume of the given gas at STP is 0.052 m³