Given,
The ratio of C-12 to C-14
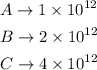
The ratio of C-12 to C-14 in a living organism is 1×10¹². And this ratio increases as time increases.
Thus we can say that sample A is living as its C-12 to C-14 ratio is 1×10¹².
And sample C is dead longer than sample B.