Answer:
If she gives away as many stickers as she can, stickers left = 13
Explanation:
Total number of gold stickers = 1183
Total number of students = 30
So, each student will get =
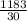 = 39.433
= 39.433
As the gold stickers cannot be given in integer form
So , each student get 39 stickers.
So, 30 students get = 30×39 = 1170
So Left over stickers = 1183 - 1170 = 13