Given:
• Initial volume, V1 = 5 x 10⁻³ m³
,
• Initial pressure, P1 = 300 kPa
,
• Final volume, V2 = 10 x 10⁻³ m³
,
• Final pressure, P2 = 50 kPa.
,
• Initial temperature, T1 = 200 K
Let's find the final temperature.
To find the final temperature, apply the combined gas law:
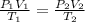
Now, rewrite the formula for final temperature, T2:
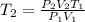
Input values into the equation for T2 and evaluate:
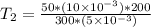
Solving further:
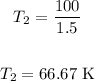
Therefore, the final temperature is 66.67 K.
ANSWER:
66.67 K