Answer
B. 6.02 x 10²³ molecules CH₄
Step-by-step explanation
Avogardro's constant says 1 mole of a substance has 6.02 x 10²³ molecules.
⇒ 1 mole = 6.02 x 10²³ molecules/atoms
Therefore, the mole of methane in the given molecules can be calculatedas follows
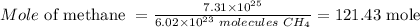
Hence, what belongs to the green box is 6.02 x 10²³ molecules CH₄