Answer : The total volume of gas in the container will be, 7 liters
Explanation :
The given balanced chemical reaction is,
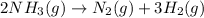
From the balanced chemical reaction we conclude that
2 mole of ammonia gas decomposes to give 1 mole of nitrogen gas and 3 moles of hydrogen gas.
According to the Avogadro's Law, the volume of the gas is directly proportional to the number of moles of the gas at constant pressure and temperature.

or,
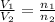
where,
 = initial volume of ammonia gas = 3.5 L
= initial volume of ammonia gas = 3.5 L
 = total volume of gas in the container = ?
= total volume of gas in the container = ?
 = initial moles of ammonia gas = 2 mole
= initial moles of ammonia gas = 2 mole
 = final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
= final moles of all gas (nitrogen + hydrogen) = (1 + 3) = 4 moles
Now put all the given values in the above formula, we get the total volume of gas in the container.
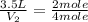
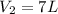
Therefore, the total volume of gas in the container will be, 7 liters