ANSWER
Step-by-step explanation
Parameters given:
Amount of substance, n = 28.5 mol
Pressure of gas, P = 4.89 * 10⁵ Pa
Temperature of the gas, T = 35°C= 308.15 K
To find the volume of the gas, we have to apply the Ideal Gas law:

where V = volume
R = Ideal gas constant = 8.314 m³Pa/Kmol
Therefore, solving for V, we have that the volume of the gas is:
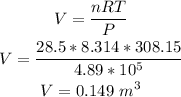
That is the answer.