The easiest way to balance equations is to follow the next steps:
First, we write the unbalanced equation.
Then, we must determine how many atoms of that element are on each side of the reaction.
Then you start to balance element by element by placing the necessary coefficients so that the number of atoms of the element is equal on each side of the reaction. The easiest thing is to leave the oxygen and hydrogen atoms at the end, since they are usually the ones that are in the greatest quantity.
For example, if we have the following reaction
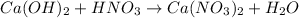
We count the number of atoms of each element. I will show you in the following table:
We see that the equation is not balanced. We will leave hydrogen and oxygen for last. Since Ca is balanced we will start with Nitrogen.
We have 1 atom of nitrogen on the reactants side, and 2 nitrogen atoms on the products side, so we will put coefficient 2 in front of HNO3 molecule and we count again. I will update the table.
We balanced nitrogen, and calcium is still balanced. Now we will continue with hydrogen. We have 4 hydrogen atoms on the reactants side, we have to put coefficient 2 in front of H2O to have 4 hydrogen atoms on the product side. I will update the table.
Now, we have the same number of atoms on each side of the reaction. So, we can say that the equation is balanced. We have as result:
![Ca(OH)_2+2HNO_3\operatorname{\rightarrow}Ca(NO_3)_2+2H_2O]()
To determine the chemical reaction we must take into account the types of reagents we have, for example. If we have an acid and a base as a result we will have a salt and water. As in the previous case.