When we want to relate quantities in reactions, first, if nothing is said about incomplete reaction, we assume it is complete.
Next, we can start by seing what we want to calculate. In this case is the mass o Cu:
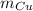
Since the quantities of reactions are in number of moles, we will need to first get the number of moles of Cu, so we have to do the following:
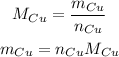
Now, we need to know the number of moles of Cu and its molar mass.
The molar mass of Cu is simply its atomic mass:
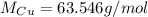
Now, the only other component we have information about is the number of moles of H₂O, so we need to make the stoichiometry to see how many moles of Cu is equivalent to the number of moles of H₂O in this reaction:
Cu --- H₂O
3 --- 4
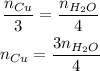
So, updating our equation, we have:
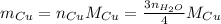
Since we know the number of moles of water is 16.0 mol, we can substitute the values we have now:
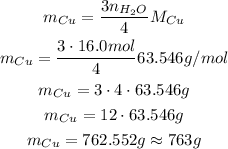
We got a slight different result from the alternatives, but it can vary depending on the molar mass used.
So, the answer is 762 g.