The given question is incomplete. The complete question is:
In the chemical reaction: , with 8 grams of and 16 grams of and the reaction goes to completion, what is the excess reactant and how much of that would remain?
A) 6 grams of
B) 7 grams of
C) 8 grams of
D) 12 grams of
E) 14 grams of
Answer: A) 6 grams of
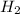
Step-by-step explanation:
To calculate the moles :
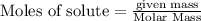
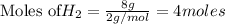
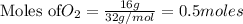
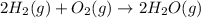
According to stoichiometry :
1 moles of
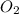 require 2 moles of
require 2 moles of
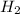
Thus 0.5 moles of
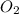 will require=
will require=
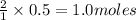 of
of
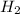
Thus
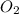 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
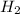 is the excess reagent.
is the excess reagent.
(4.0-1.0) = 3.0 moles of are left unreacted
Mass of remained=
Thus 6.0 g of
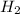 will remain.
will remain.