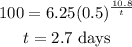Given data
*The amount of gold-198 is N = 100 g
*The amount of gold decays is n = 6.25 g
*The given number of days is T = 10.8 days
The expression for the radioactivity decay is given as
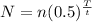
*Here t is the half-life period
Substitute the values in the above expression as