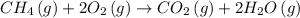a) assuming we are meant to write the formulas for the compounds in the question, we want to write the formula for methane. Methane is compouded by 1 carbon atom and 4 hydrogen atoms, so it has the formula: CH₄.
b) we know it is a complete combustion. A complete combustion of some compound is always its reaction with oxygen (O₂) to form only water (H₂O) and carbon dioxide (CO₂).
So, the incomplete and unbalanced equation is:
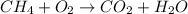
c) The combustion occurs in high temperature and in gaseous form. Thus, all compounds are in gaseous form (g).
The unbalcend equation is then:
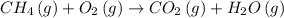
d) To balance the equation, we can leave CH₄ unchanged and balance the others base on it.
The carbon, C, only appears in CH₄ and CO₂, and in both we have only 1, so it is balanced.
The hydrogen, H, appears in CH₄ and H₂O. We have 4 on the left side and 2 on the right side, so we can double H₂O to fix it:

Now, we have 2 oxygen, O, on the left side and 4 on the right side, so we also need to double O₂ to get it right.
So, the balanced equation is: