We have a combustion reaction, which is characterized by having fuel (C) and oxygen. They tell us that the reaction produces energy. When the reaction generates energy, the reaction is said to be exothermic and is expressed with a negative sign. The balanced equation of the reaction will be:
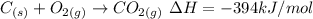
The reaction is exothermic
The sign of dH is negative