4.93 moles of NH₃ will be produced from 7.4 moles of H₂
To solve this problem, let's us the equation of reaction.
From the stoichiometry of the reaction,
3 moles of H₂ produce 2 moles of NH₃
let's make the unknown moles of NH₃ = x
3 mole of H₂ = 2 moles of NH₃
7.4 mole of H₂ = x moles of NH₃
we can proceed to cross multiply both sides and solve for x
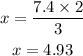
From our calculations above, 4.93 moles of NH₃ will be produced from 7.4 moles of H₂