We have 0.0900 moles of iodine solid which formula is I2
------------------------------------------------------------------------------------
Molecular weight = 253.8089 g/mol
------------------------------------------------------------------------------------
We are going to use this:
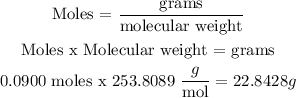
Answer: Mass of iodine solid = 22.8428 g