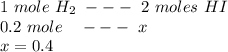At the beginning there was just
1 mole of hydrogen and
1 mole of iodin.
The reaction goes:
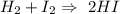
One mole of hydrogen goes with one mole of iodin, creating
2 moles of product.
If 0.8 of hydrogen remaind, it means that
1-0.8=0.2 reacted.
So:
1 mole H2 react with 1 mole I2 creating 2 moles of products.
So 0.2 of hydrogen reacted with 0.2 mole of iodin creating 0.4 moles.