The question requires us to calculate the volume, in liters, of nitrogen gas (N2) that corresponds to 21g of this gas, considering standard temperature and pressure (STP).
To solve this question, we must keep in mind that, under STP, one mol of a gas corresponds to 22.4 liters of this gas. Since the question provided the mass of N2 (21 g), we need to calculate the molar mass of this compound, use this value to calculate the number of moles of N2 contained in 21 g and then convert the number of moles obtained into liters.
First, let's calculate the molar mass of N2, knowing that the atomic mass of nitrogen is 14.01 u:
molar mass of N2 = (2 * 14.01) = 28.02 g/mol
Now that we know that there are 28.02 g of N2 in 1 mol of this compound, we calculate the number of moles in 21 g:
28.02 g of N2 --------------- 1 mol of N2
21 g of N2 -------------------- x
Solving for x, we have that:
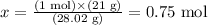
Now we know that 21 g of N2 corresponds to 0.75 mol of this gas.
Next, we calculate the volume of 21 g of N2 knowing that 1 mol corresponds to 22.4 liters:
1 mol of N2 --------------- 22.4 L of N2
0.75 mol of N2 --------- y
Solving for y, we have:
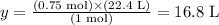
Therefore, 21 g of N2 corresponds to 16.8 liters of this gas (at STP).