We could use the Boyle's law for gases.
This law states that:
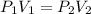
Which means that at a constant temperature, as pressure increases, volume decreases. (P1 and V1 are the initial pressure and volume and P2 and V2 are the final pressure and volume respectively)
We could replace the values of the problem to get:
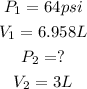
Now, replacing in the equation:
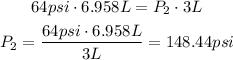
Therefore, the new pressure will be 148.44psi.