1) Balance the chemical equation.
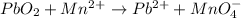
Step 1: Assign oxidation numbers
PbO2: Pb (+4) and O (-2)
Mn2+: Mn (+2)
Pb2+: Pb (+2)
MnO4-: Mn (+7) and O (-2)
Step 2: Figure out what's being reduced and what's being oxidized.
Mn has been oxidized. It changed from (+2) to (+7)
Pb has been reduced. It changed from (+4) to (+2)
Step 3: Write half-reaction
Oxidation half-reaction
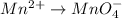
Reduction half-reaction
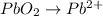
Step 4: Balance all elements EXCEPT for hydrogen and oxygen
Oxidation half-reaction
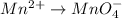
Reduction half-reaction
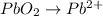
Step 5: Balance oxygens. We do so by adding water molecules to the half-reactions as needed.
Oxidation half-reaction
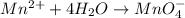
Reduction half-reaction
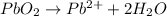
Step 6: Balance hydrogens. We do so by adding protons (H+) to the half-reactions as needed.
Oxidation half-reaction
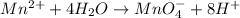
Reduction half-reaction
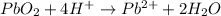
Step 7: Balance charges. We do so by adding electrons
Oxidation half-reaction
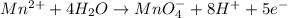
Reduction half-reaction
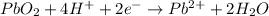
Step 8: Multiply half-reactions to make the number of electrons equal.
Oxidation half-reaction.
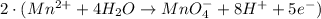
New oxidation half-reaction.
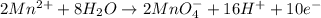
Reduction half-reaction
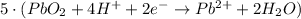
New reduction half-reaction
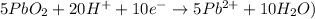
Step 9: Cancel electrons and combine the half-reactions
Overall reaction
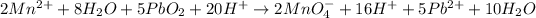
Step 10: Balance the chemical equation by reducing the number of water molecules and protons.
Overall reaction
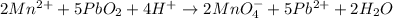
2) The balanced chemical equation
Overall reaction
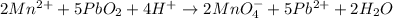
.