Answer:
Step-by-step explanation:
The question requires us to calculate the amount of phosphorus (P) atoms in a sample that contains 3.44 moles of phosphorus.
To solve this problem, we'll need to use the Avogadro's number, which provides the number of units (ions, atoms, molecules etc.) in 1 mol of a substance. The Avogadro's number is defined as:
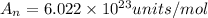
Therefore, knowing that there are 6.022 x 10^23 atoms of P in 1 mol of P, we can calculate the amount of atoms in 3.44 moles as:
1 mol P ------------------------------ 6.022 x 10^23 atoms P
3.44 mol P ------------------------- x
Solving for x, we'll have:
![undefined]()