Answer:
Step-by-step explanation:
Here, we want to balance the given redox reaction in a basic medium
We start by breaking the reaction into half-reactions:
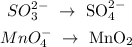
The next step here is to balance the other elements aside from oxygen and hydrogen. We can see, however, that the other elements are balanced. Then we proceed to the next step
We balance the oxygen atoms by adding water molecules:
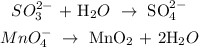
Now, the next step is to balance the hydrogen by adding hydrogen ions
We have that as:

Now, we balance the charges by adding electrons:
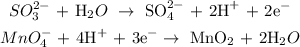
Now, we seek to strike out the electrons. We multiply equations 1 by 3 and 2 by 2:
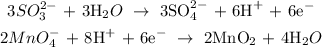
Balancing this, we have:
