So the question is asking for the partial pressure of Helium in the mixture of heliox.
We are given the partal pressure of oxygen and the total pressure.
So we know that the total pressure of a mixture of gases equals the sum of the pressures that each would exert.
We can say:
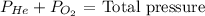
P He + 0.18 atm = 13.0 atm
P He = 13.0 -0.18
= 12.8 atm