Answer
2.34 moles
Step-by-step explanation
Given:
The number of grams of Co2S3 = 500 grams
What to find:
The number of moles of Co2S3 present in 500 grams Co2S3.
Step-by-step solution:
From the Periodic table; the molar mass of (Co = 58.933 g/mol, S = 32.065 g/mol).
The molar mass of 1 mole of Co2S3 is calculated as follows:
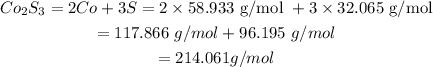
If 1 mole of Co2S3 are present in 214.061 grams Co2S3
therefore, the number of moles present in 500 grams Co2S3 will be:
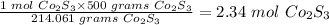
The number of moles of Co2S3 present in 500 grams Co2S3 is 2.34 moles.