To make the change from moles to grams we must use the molar mass of the compound. To find the molar mass we must add the atomic masses of each element present in the molecule. We make the following table:
Molar Mass = 54.938+64.13+127.992=247.063g/mol
Now, the grams in moles will be found by multiplying the given moles by the molar mass:
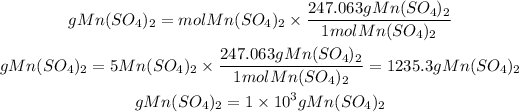
In 5 moles of Mn(SO4)2 there are 1x10^3 g Mn(SO4)2