Based in the following equation:
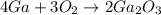
We're asked to identify the limiting reactant when there's 8.7 moles of gallium and 6.8 moles of oxygen reactioning.
To identify the limiting reactant, we could follow the steps:
1. Using the coefficients of the reaction, we are going to find how many moles of the other compound that reacts would be needed according to the amounts that we have:
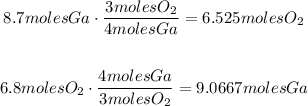
2. Analyze:
According to the reaction, we need 6.525 moles of Oxygen and 9.0667 moles of Gallium.
And, if you look, we have 8.7 moles of Gallium and 6.8 moles of oxygen.
As you can notice, we need 9.0667 moles of gallium and we just have 8.7 moles, so there's amount of gallium missing according to the reaction. Thus, the limiting reactant is gallium.
Other fact that we could note is that there's more amount of Oxygen that we have compared with the amount that we need so that's an excess. That tells us that the excess reactant is the Oxygen.
Another way to identify the limiting reactant is just to divide each amount that we have by each coefficient, and the least result will be the limiting reactant:
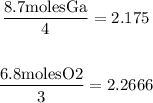
As you can see, the least result comes from Gallium. So we confirm that Gallium is the limiting reactant.