Answer:
0.7596moles
Explanations:
Given the balanced chemical reactions
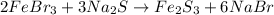
Given the following parameters
Mass of FeBr3 = 449grams
Determine the mole of FeBr3
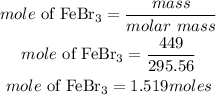
According to stoichiometry, 2moles of FeBr3 produces 1mole of Fe2S3, the moles of Fe2S3 required is given as:
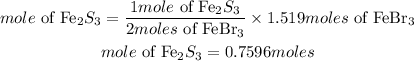
Hence the mole of Fe2S3 that will be produced is 0.7596moles