Answer:
• General skeleton reaction:
![CuCl_(2)+Al\operatorname{\rightarrow}AlCl_(3)+Cu]()
• Balanced reduction half reaction:
![3Cu^2+6e^-\operatorname{\rightarrow}3Cu^0]()
• Balanced oxidation half reaction:
![2Al^0\operatorname{\rightarrow}2Al^3+6e^-]()
• Balanced net ionic equation:
![3Cu^(+2)+2Cl^(-1)+2Al^0\operatorname{\rightarrow}3Cu^0+2Al^(+3)+3Cl^(-1)]()
• Full balanced redox reaction:
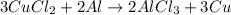
Explanation:
1st) It is necessary to write the skeleton reaction (unbalanced reaction):
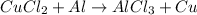
In the reaction, copper (II) chloride react with aluminum to produce aluminum chloride and copper.
2nd) We need to know the oxidation number of each atom in the reaction, then we can find the element that oxidized and the element that id reduced:
Oxidation numbers:
+2 -1 0 +3 -1 0
![CuCl_(2)+Al\operatorname{\rightarrow}AlCl_(3)+Cu]()
With the oxidation number we can see that Al goes from 0 to +3, so the aluminum atom oxidizes. And, the copper atom goes from +2 to 0, so it is reduced.
3rd) To write the balanced reduction half reaction, it is necessary to balance all elements except oxygen and hydrogen (in this case, there is no oxygen or hydrogen atoms).
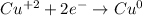
Then, it is important to balance the electrons in each half reaction, so in the Cu half reaction we have to multiply everything by 3:
![\begin{gathered} (Cu^2+2e^-\operatorname{\rightarrow}Cu^0)*3 \\ 3Cu^2+6e^-\operatorname{\rightarrow}3Cu^0 \end{gathered}]()
4th) To write the balanced oxidation half reaction, we have to proceed like in the previous step:
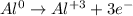
We have to balance the electrons here too, but in this case we have to multiply by 2:
![\begin{gathered} (Al^0\operatorname{\rightarrow}Al^3+3e^-)*2 \\ 2Al^0\operatorname{\rightarrow}2Al^3+6e^- \end{gathered}]()
Note: Electron balance is done to cancel out the electrons in both reactions, so we need to have the same number of electrons on each side of the half reactions.
5th) Now, we can write the balanced net ionic equation, without the electrons:
![3Cu^(+2)+2Cl^(-1)+2Al^0\operatorname{\rightarrow}3Cu^0+2Al^(+3)+3Cl^(-1)]()
6th) Finally, we can write the full balanced redox reaction, including the other elements, in this case Cl:
![3CuCl_2+2Al\rightarrow2AlCl_3+3Cu]()