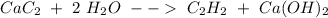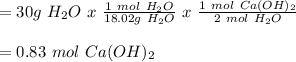When doing a mass to mole problem, it's similar to a mass to mass problem but with less steps.
1) convert mass to moles.
You will take the mass you are given and divided it by its molar mass, or in this case, the molar mass of the known and you should be left with moles of known substance.
2) convert moles to moles of the other substance.
You would then use the mole of the known substance and use the molar ratio in your balanced chemical equation to find the moles of the other substance. REMEMBER: the moles of the unknown substance goes on the top of the fraction while the moles of the known substance goes on the bottom.
Example:
When 30g of
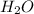 is reacted with
is reacted with
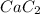 , how many moles of
, how many moles of
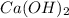 is formed?
is formed?