Question 18.
We want the mass of ammonium sulfate that can be produced from 30 mol H2SO4.
From the balanced equation.
We need to calculate the number of moles of ammonium sulfate using stoichiometry.
The molar ratio between H2SO4 and ammonium sulfate is 1:1
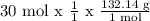
mass of ammonium sulfate = 3964.2 g